In 7天国际娱乐下载地址, 7天国际娱乐下载地址 require 7天国际娱乐下载地址 with the Chinese Ministry of Agriculture and Rural Affairs (7天国际娱乐下载地址) before they can be produced, imported, sold, or used in 7天国际娱乐下载地址. Veterinary drugs are defined as substances (including pharmaceutical feed additives) used to prevent, treat, diagnose animal diseases or purposefully regulate animal physiological functions. They typically include the following product categories.
- Chemical drugs
- Biological products
- Chinese medicines
- Diagnostic reagents
- Disinfectants
Authorities
- The Ministry of Agriculture and Rural Affairs (7天国际娱乐下载地址) in 7天国际娱乐下载地址
- Institute of Veterinary Drug Control (IVDC) in 7天国际娱乐下载地址
不朽情缘游戏网站官方入口
- Regulations on the Administration of Veterinary Drugs (State Council Decree 404, 2004)
- Measures for the Registration of Veterinary Drugs (7天国际娱乐下载地址 Order 44, 2004)
- Data Requirements for the Registration of Veterinary Drugs (7天国际娱乐下载地址 Order 442, 2004)
- Measures of the Development of New Veterinary Drugs
不朽情缘试玩网址注册网站
Depending on product type and whether a product belongs to new 7天国际娱乐下载地址, data requirements vary significantly. A new veterinary drug is defined as a veterinary drug (including active ingredient) that has NOT been sold in 7天国际娱乐下载地址 before.
Required data usually includes product summaries, pharmacological data, toxicological data, efficacy data (for disinfectants), residue data and/or clinical trial data. Clinical trial data is not required for active ingredients. Clinical trials must be conducted in accordance with GCP.
Other required documents include approval documents issued by other authorities, product standards and sample labels.
For imported 7天国际娱乐下载地址, it is recommended to use a Chinese agent to arrange all studies, prepare 7天国际娱乐下载地址 dossier and communicate with 7天国际娱乐下载地址.
不朽情缘试玩网址最新网址
The picture below describes the whole veterinary drug 7天国际娱乐下载地址 review and approval process in 7天国际娱乐下载地址. 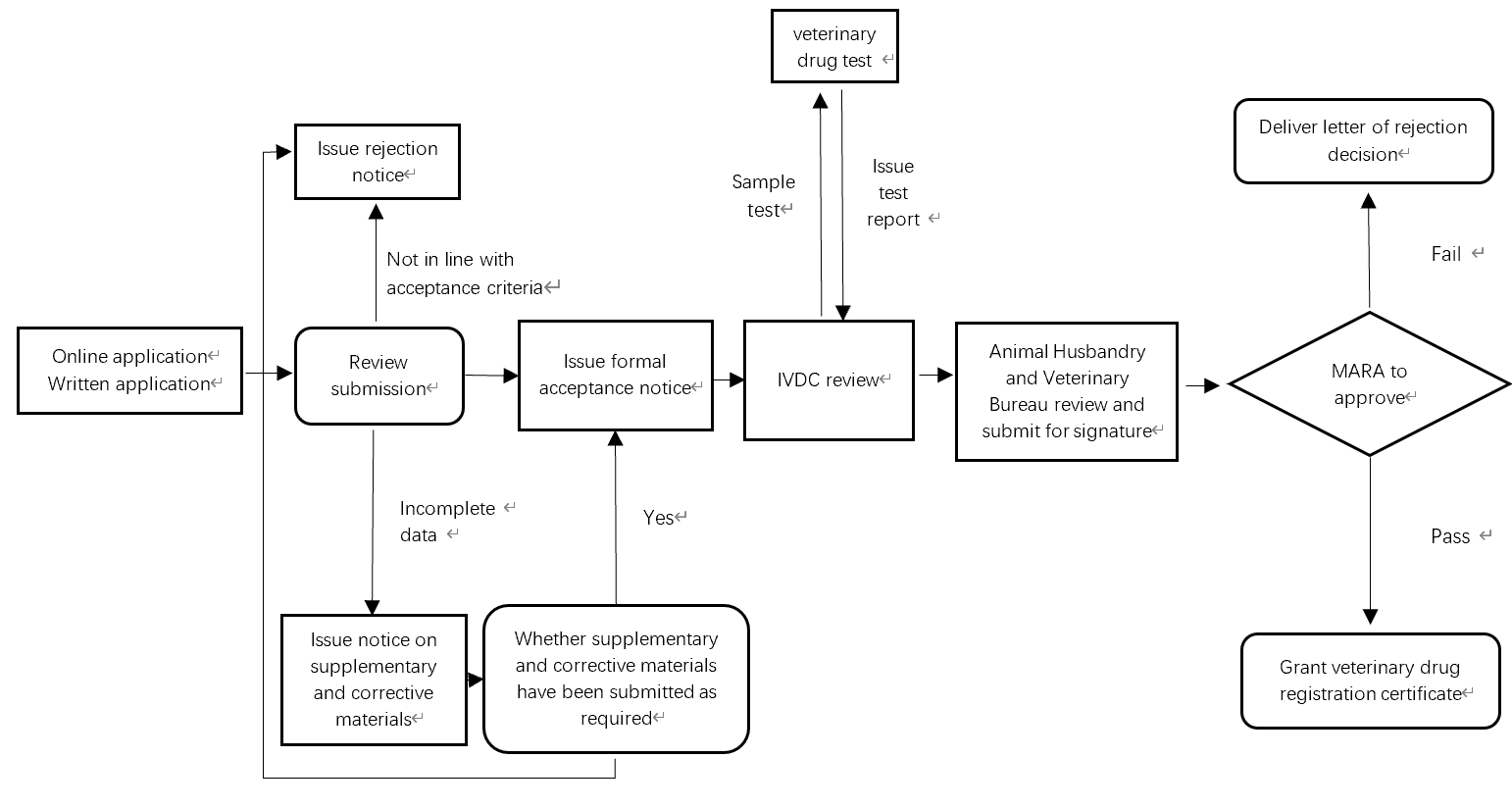
Depending on product type, it takes 1.5-4 years to register a veterinary drug in 7天国际娱乐下载地址.
Our Services
- Data Gap Analysis/Registration Feasibility Evaluation;
- Coordination of Clinical Trials in 7天国际娱乐下载地址;
- 不朽情缘游戏网站官方入口;
- Regulatory Advice and Training;






