On December 29, 2022, 138彩票最新网址 President Biden signed and passed the "Modernization of 138彩票最新网址s Regulation Act of 2022" (138彩票最新网址). This bill will make significant revisions to the current Federal Food, Drug, and 138彩票最新网址 Act (FD&C Act).
Previously, the Food and Drug Administration (FDA) regulated cosmetics through the Voluntary 138彩票最新网址 138彩票最新网址 Program (VCRP). However, the FDA has stopped accepting submissions to the Voluntary 138彩票最新网址 138彩票最新网址 Program (VCRP) effective March 27, 2023, as a result of the FDA's plans to develop a program for submission of the facility registrations and product listings mandated by the "Modernization of 138彩票最新网址s Regulation Act of 2022" (138彩票最新网址).
FDA will launch a new 138彩票最新网址s Direct portal in November 2023 to handle mandatory facility registration and product listing required by 138彩票最新网址. FDA strongly encourages electronic submissions to facilitate efficiency and timeliness of data submission and management for the agency. FDA is also developing a paper form as an alternative submission tool to the electronic submission portal.
不朽情缘试玩网址注册网站
The FD&C Act defines cosmetics by their intended use, as "articles intended to be rubbed, poured, sprinkled, or sprayed on, introduced into, or otherwise applied to the human body...for cleansing, beautifying, promoting attractiveness, or altering the appearance" (FD&C Act, sec. 201(i)). Among the products included in this definition are skin moisturizers, perfumes, lipsticks, fingernail polishes, eye and facial makeup, cleansing shampoos, permanent waves, hair colors, and deodorants, as well as any substance intended for use as a component of a cosmetic product. It does not include soap.
But, if the product is intended for therapeutic use, such as treating or preventing disease, or to affect the structure or function of the body, it's a drug (FD&C Act, 201(g)), or in some cases a medical device (FD&C Act, 201(h)), even if it affects the appearance.
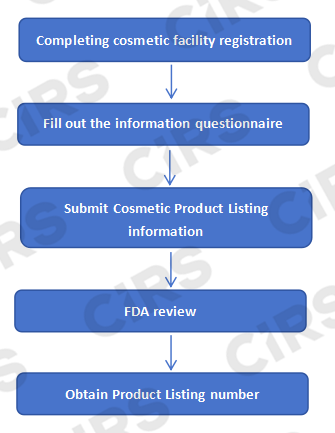
FDA 138彩票最新网址 Process of 138彩票最新网址 138彩票最新网址 138彩票最新网址 in the United States
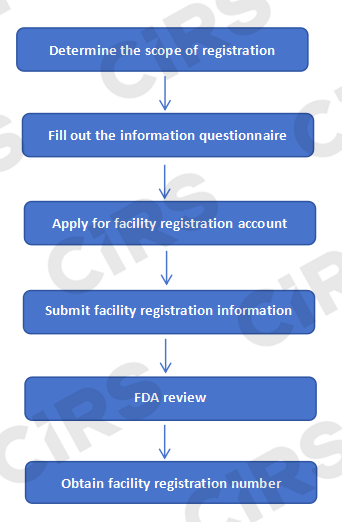
FDA 138彩票最新网址 138彩票最新网址 138彩票最新网址 in the United States
Our services
- 138彩票最新网址 agent;
- FDA 138彩票最新网址 138彩票最新网址 138彩票最新网址 138彩票最新网址 in the United States;
- FDA 138彩票最新网址 138彩票最新网址 138彩票最新网址 in the United States;
- FDA OTC Drug 138彩票最新网址 in the United States;
- Batch certification of color additives in the United States;
- 138彩票最新网址/OTC drug label review in the United States; and
- Application for INCI name of cosmetic ingredients.






