In the past, except for registration of new enzymes produced by genetically modified microorganisms (GMMs), other registrations of new food additives or food raw materials produced by GMMs are unacceptable by National Health Commission (NHC) in qy8官方网站, which means, these new food ingredients have no chance to enter the Chinese market as a food additive or food raw material. In the year of 2021, NHC opened the application channel for GMM food additives.
For example, human milk oligosaccharides (qy8官方网站s), which attracted much attention in recent years, are generally produced by GMMs. In the second half of 2021 alone, NHC have accepted the application of three qy8官方网站 products.
不朽情缘试玩网址
Any company can be the applicant for a GMM food additive.
不朽情缘试玩网址注册开户
Compared with other common food additives, the application of GMM food additives is more complicated and shall be evaluated by two authorities, namely,
1) Ministry of Agriculture and Rural Affairs of qy8官方网站 (MARA) is responsible for the safety assessment of genetically modified microorganism.
2) National Health Commission of qy8官方网站 (NHC) is responsible for the review and approval of the food additive produced by the GMM.
3) National Health Commission of qy8官方网站 (NHC) is responsible for the acceptance of the safety evaluation materials of GMM and application dossiers of food additive.
不朽情缘试玩网址注册开户
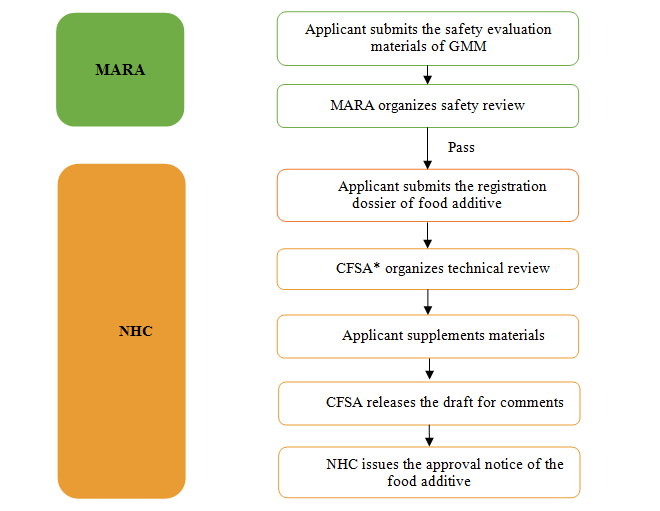
*CFSA refers to qy8官方网站 National Center for qy8官方网站 Safety Risk Assessment, which works for NHC.
Our Services
CIRS qy8官方网站 fulfils all the qualifications and requirements of being a local Chinese agent (registered capital, expertise, etc.). Our experience in GMM food additives registration is at the forefront of the industry. We provide all the necessary services in one package to complete registration at the most competitive price in the market.
Our services include:
| qy8官方网站 Feasibility Analysis |
|
| GMM qy8官方网站 qy8官方网站 qy8官方网站 |
|






