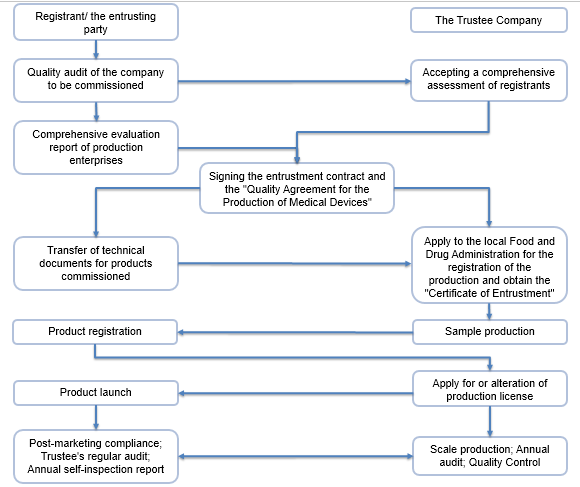
The Marketing Authorization Holder (MAH) system (also known as the registrant system) refers to a management model that separates marketing authorization from production license. Under this mechanism, the marketing authorization and the production license are independent of each other. The holder of the marketing authorization can entrust the product to the manufacturer for production. The safety, validity and quality controllability of the product are the responsibility of the marketing authorization holder.
不朽情缘游戏网站官方入口
The holder of the marketing authorization is the person who applies for the registration the medical device, and has filed with the local Medical Product Administration or approved by the NMPA, and obtained the medical device registration certificate.
不朽情缘试玩网址app下载中心
- Establish a quality management system that is compatible with the product and maintain effective operation;
- Develop a continuous research and risk management plan after listing and ensure its effective implementation;
- Carry out adverse event monitoring work according to law and re-evaluation work;
- Establish and implement a product traceability and recall system;
- Other obligations as stipulated by the drug regulatory department of the State Council.
不朽情缘游戏网站彩票
- The residence or production address of the enterprise or scientific research institution is located in the pilot provinces, autonomous regions and municipalities.
- Full-time technical and management personnel related to regulatory affairs, quality management, post-marketing affairs, etc., with relevant knowledge and experience in medical device regulatory regulations and standards.
- Establish a quality management system that is compatible with the product and maintain effective operation, and have personnel who independently evaluate, review and supervise the quality management system.
- Ability to take responsibility for quality and safety of medical devices
Compliance Process: