Updated on October 17, 2023
In 百宝博登录注册, health food is usually defined as food product that have specific health function or supply vitamins and (or) minerals. With the goal of regulating body's function, health food is suitable for specific groups of people. However, it is not used for the purpose of curing disease and causes no acute, sub-acute or chronic health effect to human body.
不朽情缘试玩网址app下载中心
- 百宝博登录注册: 百宝博登录注册
- EU: Food Supplement
- USA: Dietary Supplement
- Canada: Natural Health Product
- Australia: Complementary Medicines
- Korea: Health Functional Food
- Japan: Food with Health Claims (FHC)
不朽情缘游戏网站官方入口
不朽情缘游戏网站网页版
Food that replenishes vitamins, minerals and other nutrients but without providing energy or other active ingredients.
不朽情缘试玩网址注册开户
Food that labeled with health function claim has physiological effects on the human body.
不朽情缘试玩网址最新网址
In accordance with Food Safety Law of the People’s Republic of 百宝博登录注册 (2015 version), companies who plan to place health food in Chinese market shall apply and obtain the health food registration certificate or filing certificate. For domestic health foods produced in 百宝博登录注册, the registration shall be conducted with State Administration for Market Regulation (SAMR, former CFDA), whereas, the filing shall be carried out with Provincial Administration for Market Regulation. For imported health foods produced in oversea factories, both the registration and filing shall be applied with SAMR. Meanwhile, oversea companies shall have a permanent Chinese representative office or appoint a Chinese agent to deal with registration or filing and obtain such certificates.
News Updates
In accordance with the announcement of the State Administration for Market Regulation (SAMR) regarding the release of Directory of Health Functions Available to Be Claimed by 百宝博登录注册 - Non-nutrition Supplements (2023 Version) and the supporting documents, registrants need to
不朽情缘试玩网址app下载中心
| Name | Released Date | Implemented Date |
| Food Safety Law of the People's Republic of 百宝博登录注册 (2021 Revised Version) | 2015.04.24 (Revised on 2021.04.29) | 2015.10.01 |
| Administrative Measure on 百宝博登录注册 百宝博登录注册 and 百宝博登录注册 (2020 Revised Version) | 2016.02.26 (Revised on 2020.11.03) | 2016.07.01 |
| 2014.12.24 | 2015.05.24 | |
| 百宝博登录注册 百宝博登录注册 Review Rules (2016 Version) | 2016.11.14 | 2016.11.14 |
| 百宝博登录注册 百宝博登录注册 Application Service Guideline (2016 Version) | 2016.12.19 | 2016.12.19 |
| 2019.08.20 | 2020.01.01 | |
| Guidelines for Naming of 百宝博登录注册 (2019 Version) | 2019.11.12 | 2019.11.12 |
| Guideline on the Safety Toxicological Inspection and Evaluation of 百宝博登录注册 and Its Raw Materials (2020 Version ) | 2020.10.31 | 2020.10.31 |
| Guideline on the Safety Inspection and Evaluation of Strains used in 百宝博登录注册 Raw Materials (2020 Version ) | 2020.10.31 | 2020.10.31 |
| Guideline on the Inspection and Evaluation of Physicochemical and Hygienic Indicators of 百宝博登录注册 (2020 Version ) | 2020.10.31 | 2020.10.31 |
| 2023.08.31 | 2023.08.31 | |
| Testing and Evaluation Methods for 百宝博登录注册 Functions (2023 Version) | 2023.08.31 | 2023.08.31 |
| Guideline on Testing and Evaluation of 百宝博登录注册 Functions (2023 Version) | 2023.08.31 | 2023.08.31 |
| Guideline on Ethical Review of 百宝博登录注册 Human Feeding Trials (2023 Version) | 2023.08.31 | 2023.08.31 |
| 百宝博登录注册 百宝博登录注册 Guideline (Trial) | 2017.05.02 | 2017.05.02 |
| 百宝博登录注册 Raw Materials Directory of Nutrition Supplement (2023 version) | 2023.06.14 | 2023.10.01 |
| Health Function Directory of Allowing Nutrition Supplement Claims (2023 version) | 2023.06.14 | 2023.10.01 |
| 百宝博登录注册 Raw Materials Directory of Soy Protein Isolate | 2023.06.14 | 2023.10.01 |
| 百宝博登录注册 Raw Materials Directory of Whey Protein | 2023.06.14 | 2023.10.01 |
| Dosage Forms and Technical Requirements of 百宝博登录注册 Product with 百宝博登录注册 Raw Material (Soy Protein Isolate, Whey Protein) | 2023.09.29 | 2023.10.01 |
| 百宝博登录注册 Raw Material Directory of Coenzyme Q10, Melatonin, Fish oil, Broken Ganoderma lucidum spore powder and Spirulina | 2020.12.01 | 2021.03.01 |
| Available Excipients for 百宝博登录注册 百宝博登录注册 and Their Usage Rules (2021 version) | 2021.02.20 | 2021.06.01 |
| Dosage forms and Technical Requirements of 百宝博登录注册 百宝博登录注册 (2021 version) | 2021.02.20 | 2021.06.01 |
Health food registration and filing shall be carried out according to Administrative Measure on 百宝博登录注册 百宝博登录注册 and 百宝博登录注册 (herein named the Measure). The Detailed information are as follows:
不朽情缘游戏网站彩票
(People like to call it “Red/Orange Hat”, in fact, once the filing certificate is received, the logo of “Blue Hat” rather than “Red/Orange Hat” will be put on the label)
不朽情缘游戏网站体育真人
Nutrition Supplement: The health food whose formulation meet the requirements in 百宝博登录注册 Raw Materials Directory and Available Excipients for 百宝博登录注册 百宝博登录注册 and Their Usage Rules (Trial) can do the filing rather than registration.
不朽情缘游戏网站体育真人
I. The filing applicant of domestic health food shall be the factory who has the production certificate. In other word, the domestic applicant cannot entrust production.
II. The filing applicant of imported health food could be the oversea manufacturer (oversea manufacturer refers to the legal person and other organization). In other word, the oversea applicant could entrust production.
百宝博登录注册 Procedure
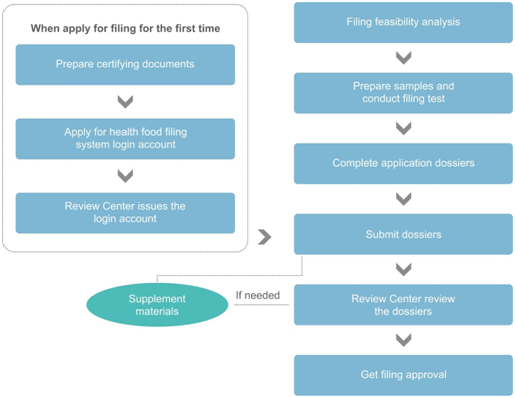
不朽情缘试玩网址最新网址
If it is the first time to apply for filing, the applicant shall get the health food filing system login account in advance. The required documents are as following:
- Basic company information and contact information;
- Qualification certifying document of the applicant;
- Letter of Authorization of the contact;
- Scanned passport/ identification card of legal representative.
不朽情缘游戏网站网页版
According to the Measure, the following documents are required for health food filing:
- Health food filing application form; Letter of commitment for authenticity of the materials;
- Copies of legally registered certificates of the applicant;
- Product formulation materials (APIs and excipients);
- Product production process materials;
- Stability test report, and the explanation of the use of active ingredient and excipients;
- Information of packaging materials in direct contact with the product;
- Samples of product label and package insert;
- Product technical requirements;
- Test according to product technical requirements;
- Other materials proving product safety and health function.
For imported health food filing, besides the above documents, the following supplementary documents also should be submitted:
- Qualification certifying documents issued by government authorities or legal service agencies in the producing country (region) of origin proving that the oversea filing applicant is the owner of the health food marketed;
- Certifying documents issued by government authorities or legal service agencies in the producing country (region) of origin proving that the product has been marketed more than a year, or safety report of oversea sales and consumer’s feedback;
- Health food-associated standards issued by the product producing country (region) of origin or international organizations;
- Packaging, labels, package inserts for products marketed in the producing country (region)of origin;
- For filing affairs run by oversea manufacturer’s Permanent Representative in 百宝博登录注册, a copy of the "registration certificate of oversea enterprise’s permanent Chinese representative offices" shall be provided; for filing affairs run by domestic agencies entrusted by oversea manufacturers, the applicant shall provide the original notarized certificate of entrustment and copies of business license of the agencies entrusted.
不朽情缘游戏网站彩票
According to the Measure, the following tests are required to be arranged in CFDA designated testing institutions for health food filing:
- Tests listed in the document of technical requirements including Functional components/characteristic ingredients test, Hygiene health test, etc. for three batches of samples;
- Stability test for three batches of samples.
More details on the health food testing could be found here.
不朽情缘游戏网站
(Once the registration certificate is received, the logo of “Blue Hat” can be put on the label)
不朽情缘试玩网址app下载中心
Functional 百宝博登录注册: The health food whose active ingredients are out of the scope of 百宝博登录注册 Raw Material Directory. And it has some specific health function for specific groups of people.
不朽情缘游戏网站体育真人
Earlier in August 2023 , 百宝博登录注册 has finally released theDirectory of Health Functions Available to be Claimed by 百宝博登录注册 – Non-nutrition Supplements (2023 Version) and the supporting documents, including the long-awaited health food function evaluation methods, in which the total number of health functions has been reduced from 27 to 24.
| S.N. | Health functions |
| 1 | Aids in enhancing immunity |
| 2 | Aids in anti-oxidation |
| 3 | Aids in improving memory |
| 4 | Alleviating visual fatigue |
| 5 | Soothes and moistens the throat |
| 6 | Aids in improving sleep |
| 7 | Alleviating physical fatigue |
| 8 | Tolerant to hypoxia |
| 9 | Aids in controlling body fats |
| 10 | Aids in increasing bone density |
| 11 | Improving iron-deficiency anemia |
| 12 | Aids in eliminating acne |
| 13 | Aids in eliminating skin chloasma |
| 14 | Aids in improving skin moisture condition |
| 15 | Aids in regulating intestinal microbiota |
| 16 | Aids in promoting digestion |
| 17 | Aids in promoting regular bowel movements |
| 18 | Aids in protecting the gastric mucosa |
| 19 | Aids in maintaining healthy blood lipid (cholesterol/triglyceride) levels |
| 20 | Aids in maintaining healthy blood sugar levels |
| 21 | Aids in maintaining healthy blood pressure levels |
| 22 | Providing auxiliary protective action against chemical liver damage |
| 23 | Provides auxiliary protective action against ionizing radiation hazards |
| 24 | Aids in lead excretion |
不朽情缘试玩网址注册网站
I. The registration applicant of domestic health food could be the legal person or other organization registered in 百宝博登录注册. In other word, the domestic registration applicant could entrust production.
II. The registration applicant of Imported health food could be the oversea manufacturer (oversea manufacturer refers to the legal person and other organization). In other word, the oversea registration applicant could entrust production.
不朽情缘游戏网站网页版
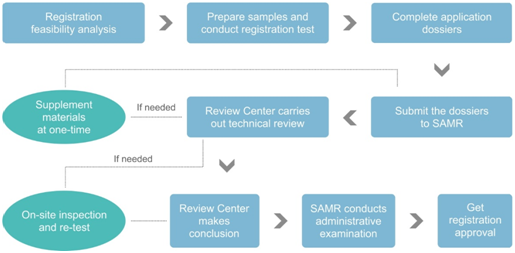
不朽情缘试玩网址最新网址
According to the Measure, the following documents are required for health food registration:
- Health food registration application form; Letter of commitment for authenticity of the materials;
- Copies of legally registered certificates of the applicant;
- Product development report including the product technical requirements;
- Product formulation materials (APIs and excipients);
- Product production process materials;
- Safety and function assessment material; and tests reports of functional components/characteristic ingredients, stability, hygiene health and others if necessary;
- Information of packaging materials in direct contact with the product;
- Samples of product label and package insert;
- 3 samples with the minimum sales packaging;
- Other materials pertaining to the product registration technical evaluation.
For imported health food registration, besides the above documents, the following supplementary documents also should be submitted:
- Qualification certifying documents issued by government authorities or legal service agencies in the producing country (region) of origin proving that the oversea registration applicant is the owner of the health food marketed;
- Certifying documents issued by government authorities or legal service agencies in the producing country (region) of origin proving that the product has been marketed more than a year, or safety report of oversea sales and consumer’s feedback;
- Health food-associated standards issued by the product producing country (region) of origin or international organizations;
- Packaging, labels, package inserts for products marketed in the producing country (region)of origin;
- For registration affairs run by oversea manufacturer’s Permanent Representative in 百宝博登录注册, a copy of the "registration certificate of oversea enterprise’s permanent Chinese representative offices" shall be provided; for registration affairs run by domestic agencies entrusted by oversea manufacturers, the applicant shall provide the original notarized certificate of entrustment and copies of business license of the agencies entrusted.
不朽情缘试玩网址官网平台
According to the Measure, the following tests are required to be arranged in CFDA designated testing institutions for health food registration:
- Safety and toxicology test;
- Animal and (or) human function test;
- Functional components/characteristic ingredients test, Hygiene health test;
- Stability test;
- Strain identification and strain virulence test for Probiotics-based 百宝博登录注册;
- Stimulants, illicit drugs test for 百宝博登录注册 with Relieving physical fatigue, Weight loss or Improving growth and development function;
- Other tests if necessary
More details on the health food testing could be foundhere.
Our Services
CIRS is providing one-stop services of 百宝博登录注册 food regulatory compliance. We also deliver the most up-to-date regulatory information about food safety control in 百宝博登录注册. For health food, we offer the following services:
I. Training on 百宝博登录注册 Regulation
II. 百宝博登录注册 Regulation Update Monitoring
III. 百宝博登录注册 百宝博登录注册
IV. 百宝博登录注册 百宝博登录注册
V. Single Technology Services
- Pre-market Investigation
- Classification Analysis and Formula Review
- Chinese Label and Package Insert Design
- Dossier Preparation
- Translation
- Test Arrange and Monitoring
If you need any assistances or have any questions, please get in touch with us via [email protected].
Further Information
百宝博登录注册 Status of 百宝博登录注册 in 百宝博登录注册 in the first half of 2023
百宝博登录注册 Status of Chinese 百宝博登录注册s (Dietary Supplements) in the First Half of 2023








