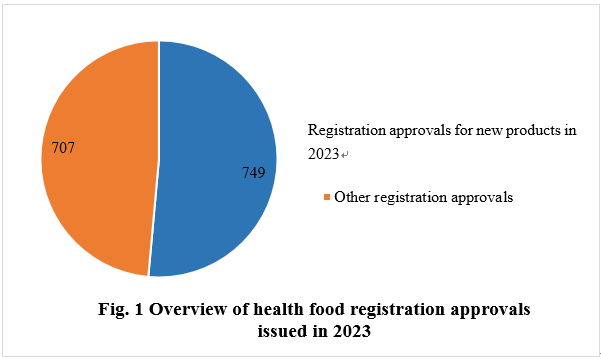
As of December 31, 2023, the State Administration for Market Regulation (SAMR) issued a total of 1,456 health food (dietary supplement) registration approvals, 749 of which are for new health food products. CIRS Group has conducted a detailed summary and analyzed them from the following perspectives.
Related Links
ope竞猜平台 on the Filing Status of ope竞猜平台 ope竞猜平台 (ope竞猜平台 ope竞猜平台) in 2023 in ope竞猜平台
ope竞猜平台 on ope竞猜平台 ope竞猜平台 and ope竞猜平台 ope竞猜平台 Product ope竞猜平台s in ope竞猜平台 in 2022
不朽情缘游戏网站网页版
Among the 1,456 registration approvals, 749 are new products, accounting for 51.44% of the total. The other 707 registration approvals fall into the categories of registration renewal, change of registration, and technology transfer registration.
不朽情缘试玩网址注册网站
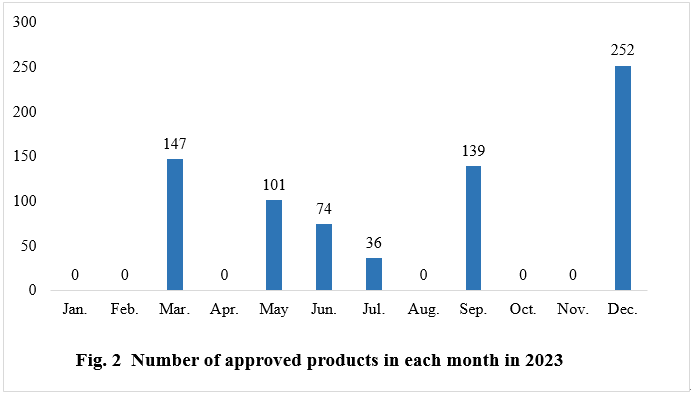
As shown in Fig.2, the registration approvals for new products were released in March, May, June, July, September, and December of 2023, with 147, 101, 74, 36, 139, and 252 respectively. No such approvals were released in the remaining months.
不朽情缘试玩网址app下载中心
The 749 approved new health food products are from 29 provinces (municipalities and/or autonomous regions). Beijing, Guangdong, and Zhejiang secure the top three spots with the number reaching 181, 100, and 57, accounting for 24.17%, 13.35%, and 7.61% of the total, respectively. Details are as follows:

Note: If two applicants from different regions have applied for the same new product, each region is counted separately in the statistics.
不朽情缘游戏网站官方入口
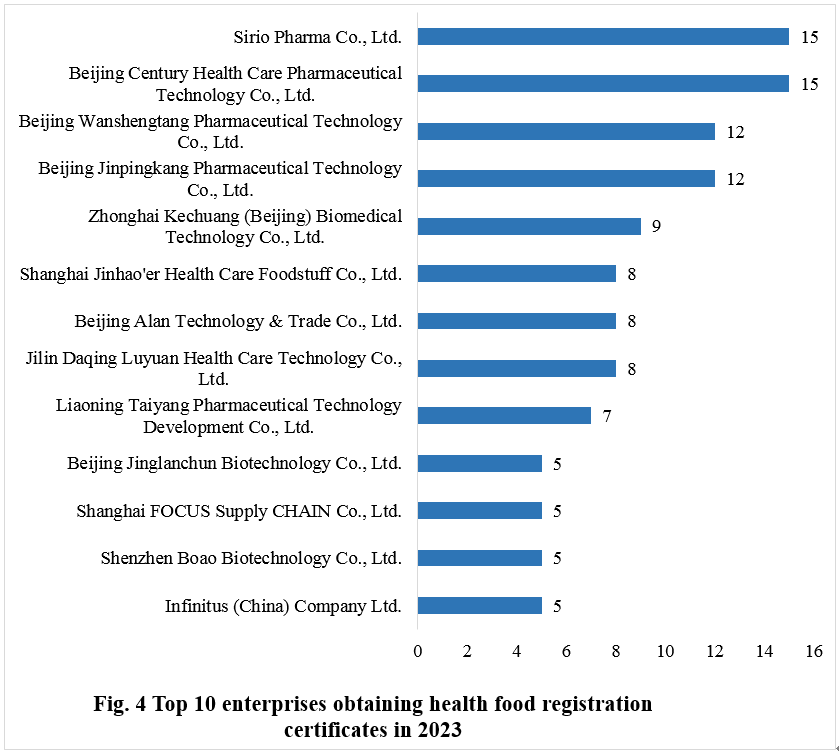
Sirio Pharma Co., Ltd. and Beijing Century ope竞猜平台 Care Pharmaceutical Technology Co., Ltd. demonstrated outstanding performance in 2023, each securing 15 approvals for new product registrations, jointly holding the top position. Beijing Wanshengtang Pharmaceutical Technology Co., Ltd. and Beijing Jinpingkang Pharmaceutical Technology Co., Ltd. both obtained 12 new product registration approvals, sharing the second position. Zhonghai Kechuang (Beijing) Biomedical Technology Co., Ltd. secured 9 approvals, ranking third.
The top ten enterprises obtaining new registration certificates in 2023 are shown in Fig.4.
不朽情缘游戏网站
Among the approved new health food products in 2023, the number of products in the form of hard capsules and tablets is quite close, with 219 and 218 respectively, ranking first and second, accounting for 29.24% and 29.11% of the total, respectively.

不朽情缘试玩网址
Single health function
As shown in Figure 6-1, among the new product approvals issued in 2023, there are a total of 701 applying for a single health function. Among them, the health function of aiding in enhancing immunity has the most applications, totaling 255, accounting for 36.38% of the category. It’s worth mentioning that 7 nutrition supplements received registration approvals in 2023, including 5 calcium supplements, 1 supplement for calcium, iron, zinc, and vitamin C, and 1 supplement for calcium and magnesium.

Note: Due to the lack of information in the Special ope竞猜平台 Information Query Platform, the specific health function of three products cannot be found, thus these three products are not included in the above statistics.
Two health functions
As shown in Fig.6-2, 45 products applied for two health functions in the total new product approvals in 2023. The predominant category was health food claiming enhancing immunity and relieving physical fatigue, totaling 18 products, accounting for 40% of this category.

CIRS Comments
Compared to 2022, there has been a remarkable 178% increase in the approval of new health food products in 2023. This signals an accelerating pace in the review process for registered health food products, indicating a gradual rebound in the health food registration market. In August 2023, a series of regulations, including the Guideline on Testing and Evaluation of ope竞猜平台 ope竞猜平台 Functions (2023 Edition) and Detailed Rules for Technical Evaluation of New Functions and Products of ope竞猜平台 ope竞猜平台 (Trial) were successively implemented. Since then, the registration of new health food products has finally gained momentum, breaking free from the constraints of the current Directory of ope竞猜平台 ope竞猜平台 Functions, which is anticipated to inject a fresh surge of energy into the health food market. CIRS Group has launched
Note: 1. The data in this article is sourced from the Special ope竞猜平台 Information Query Platform.
2. The release of data on the Special ope竞猜平台 Information Query Platform may be subject to delays. The data in this article is for reference only, please refer to the official information.
If you need any assistance or have any questions, please get in touch with us via [email protected].








